Multiple Choice
Identify the
letter of the choice that best completes the statement or answers the question.
|
|
|
1.
|
Use the phases of matter illustration below to answer questions 1 &
2.
1. Which choice above puts the illustrations in order
from least amount of energy to the greatest? a. | A,B,C,D | c. | C,A,D,B | b. | C,B,A,D | d. | D,A,C,D | | | | |
|
|
|
2.
|
Which
choice above has a definite volume, but no definite shape?
|
|
|
3.
|
Read the passage below and answer question 3.
Jackie, Johnnie, Jillian, and River went camping for the weekend. Jackie
and Jillian 1 unpacked the tent and set it up in the clearing of
the campground. Johnnie and River decided they would get the campfire started. River
2cut off some tree limbs and piled them into the fire pit. Johnnie
3 lit the match to get the logs burning. After the girls setup the tent,
they each decided to roast marshmallows with the boys. Jillian pushed a marshmallow onto the
end of a long stick and held it into the campfire. The marshmallow 4started to turn
black in color as it roasted. Johnnie accidentally dropped his marshmallow into the dirt
before getting it onto the end of the long stick. His marshmallow
5became brown in color now instead of the pure white color that marshmallows
are known for.
According to the
numbered underlined portions from the passage above, the following are physical
changes:
a. | 1,2,3 | c. | 3,4,5 | b. | 2,3 | d. | 1,2,5 | | | | |
|
|
|
4.
|
Which two
elements above are most likely to bond together based on their Bohr models
above? a. | Lithium &
Neon | c. | Neon &
Carbon | b. | Fluorine & Carbon | d. | Lithium & Fluorine | | | | |
|
|
|
5.
|
Which element
above is least likely to bond with any other elements? a. | Lithium | c. | Neon | b. | Fluorine | d. | Carbon | | | | |
|
|
|
6.
|
Referring to the Bohr models above, how many valence electrons does Carbon
have?
|
|
|
7.
|
Referring to the Bohr models above, what is the atomic number of
Fluorine?
|
|
|
8.
|
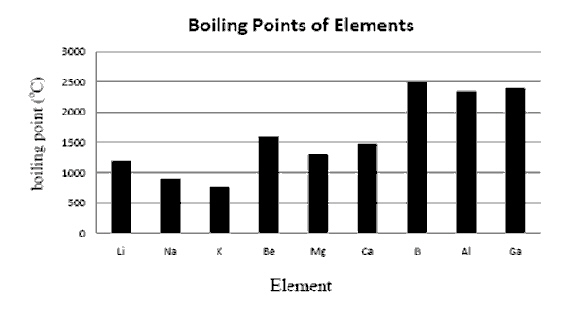 Of the three elements in the Alkali Metal
Family-group 1(Lithium-Li, Sodium-Na, and Potassium-K) which has the highest boiling
point? Of the three elements in the Alkali Metal
Family-group 1(Lithium-Li, Sodium-Na, and Potassium-K) which has the highest boiling
point?
a. | Lithium | c. | Potassium | b. | Sodium | d. | All the same | | | | |
|
|
|
9.
|
Approximately, how much higher is the boiling point of Boron (B) than Magnesium
(Mg)? a. | 1250 degrees
Celsius | c. | 2000 degrees
Celsius | b. | 2500 degrees Celsius | d. | 500 degrees Celsius | | | | |
|
|
|
10.
|
In
the graph above, the boiling point is considered the following; a. | Dependent
Variable | b. | Independent
Variable | | | | |
|